CHARLES A. TAYLOR, JR. Texas A&M University Research Station, Sonora, Texas
Abstract: Management of woody plants with the purpose of increasing herbaceous production is a goal of many resource managers. Biological control of brush through the use of goats has been a common management practice over much of west-central Texas for many years. However, refinement of “goating” is needed to provide the level of “sculpting” desired for wildlife habitat (i.e., deer) needs. Properly managed, the goat is an excellent tool for vegetation manipulation.
The invasion and increase of brush into areas previously dominated by herbaceous vegetation has been a problem on rangelands since the development of the livestock industry. This vegetation shift from grassland or grassland/savanna to shrub/woodland has been documented on most rangelands of the U.S. (McPherson and Wright 1990).
Although there is not unanimous agreement why this change took place, most authors have attributed this woody plant increase to grazing disturbance (heavy stocking) which reduced fire frequencies and improved the seed dispersal of many shrub species (Archer et al. 1988, Johnsen 1962, Wright 1972). The detrimental effects of woody plant invasion generally include reduced livestock carrying capacity (Taylor and Ralphs 1992), decreased water yield and increased erosion (Thurow and Taylor 1995), increased labor costs associated with problems of livestock management (Scifres 1980), and a loss in biodiversity (Fuhlendorf and Smeins 1997).
Management of woody plants with the purpose of increasing herbaceous production is a goal of many resource managers. Woody plant removal is usually expensive and success can be variable for most techniques. Therefore, preventive measures and management of such lands requires an understanding of the causes of the problem as well as the implementation of economic methods to shift the vegetation complex in a desired successional direction.
The kinds of expertise required to implement different brush management practices varies considerably. Mechanical control of brush requires the least amount of expertise but has high inputs of energy and costs. Chemical control requires more expertise than mechanical, and can also be expensive when applied at a broadcast level. Depending on the method used both mechanical and chemical have the potential to damage non-target species. Burning has lower levels of cultural inputs and is potentially cheaper than mechanical or chemical methods, but it requires higher levels of expertise and a commitment to long-term management
planning. Biological management of brush also requires a high level of expertise and a commitment to long-term management. It also may be the cheapest of all brush management methods, especially when used in conjunction with other methods. If improperly managed, biological control can also cause significant damage to non-target species. Obviously different kinds of brush management practices have both positive and negative aspects (Table 1).
How does brush defend itself against herbivory?
To understand how brush can be biologically managed (i.e., consumed by herbivores) is to understand that all species of brush have chemical and/or physical characteristics that can reduce the forage value of the plant. Also, many of these chemical compounds found in brush, called allelochemicals, can range from being slightly toxic or severely detrimental to herbivores, depending on a wide array of environmental factors and management practices. Generally, most browse plants do not produce sufficient quantities of allelochemicals to provide complete protection of all their tissues; however, allelochemicals occur in varying amounts in different species of plants and within different parts of the same plant.
Two examples of allelochemicals produced in important range plants are tannins and terpenoids. Tannins are produced by oak species and terpenoids are produced by juniper species. Both of these chemicals reduce the digestibility of the forage as well as the palatability and, if consumed in large enough quantities, can have negative physiological effects on the herbivore. The important point to understand about allelochemicals is that they present significant chemical barriers to herbivory and are present in most shrubs.
Herbivore strategy to cope with plant chemicals
Even though most shrubs have allelo-chemicals for plant defense, herbivores have co-evolved with these plants for thousands of years and have developed their own unique methods of dealing with them. In general, animals cope with plant defense chemicals in two ways; 1) the animal’s foraging behavior, and 2) its physiological capability.
In regards to the first strategy, herbivores may learn to avoid or minimize the use of plants containing chemical defenses. They may learn this avoidance because the plant tastes different (novel food) and they become tentative in their foraging effort or they may develop a conditioned flavor aversion for certain plants when their consumption is followed by negative gastrointestinal consequences (i.e., nausea or malaise; Fig. 1). Conditioned flavor aversion learning has been demonstrated in many herbivores including insects (Bernays and Lee 1988), monogastric mammals (Garcia 1989), and ruminant mammals (Provenza 1995).
The second strategy herbivores use to cope with plant defense chemicals is also very complex and involves many different mechanisms. These include chemical and microbiological detoxification mechanisms in the gastrointestinal tract, a host of detoxifying enzymes in the liver, and similar enzymes in all other tissues. One mechanism is the inactiv-ation of a particular allelochemical by a chemical reaction during the digestive process. For example, tannins [allelochemicals found in liveoak (Quercus virginiana)] have a strong affinity for proteins produced in the saliva of deer. As deer consume liveoak the tannins are quickly complexed with the saliva protein during the chewing process. This is an example of a “first line of defense” against these compounds.
It is beyond the scope of this paper to review the extensive literature that deals with metabolism of plant allelochemicals; however, some generalizations can be made concerning their overall fate. They are absorbed in the lipid-soluble form and are excreted as water-soluble metabolites. Therefore, metabolism of plant defense chemicals involves enzymatic reactions to convert fat-soluble substances to water-soluble compounds. These metabolic processes, known as biotransformation, may either increase or decrease the toxicity of the ingested allelochemical (Cheek and Shull 1985); because of this, they have a profound effect on the ability to manage brush by biological means. For example, goats have a greater tolerance for the class of chemicals (terpenoids) found in juniper than other domestic livestock (Straka 1993). Insect herbivores are notorious for feeding on plants with high concentrations of allelochemicals. This kind of knowledge is critical for the development of biological management practices on brush.
Biological control of brush found on rangelands by the classical approach ( i.e., introduction of alien control agents such as scale insects) will not be discussed in this paper. Most brush species in Texas are native rather than introduced, thus reducing the effectiveness of managed insect herbivory on brush. Therefore, this discussion will focus on domestic livestock as biological control agents.
Biological brush management with domestic livestock
Rangelands consist of mixtures of vegetation communities containing a wide array of plant species. These communities result from differences in the underlying soil, rainfall, aspect and the impact of humans and grazing management. Because of this variety in plant composition on rangelands, herbivores have many foraging choices to make.
Management of brush by browsing animals “is applied to achieve a desired directional succession in vegetation composition; it is one form of planned environmental manipulation by controlled grazing” (Vallentine 1989). However, before effective brush management can be accomplished by browsing animals, a basic understanding of the selective foraging process must be understood.
Different species of grazing animals have different forage preferences. Cattle are primarily grass eaters but do consume some forb and browse species. Goats consume primarily browse and grass but they will also select forbs. Sheep generally consume mostly grass and forbs but under certain conditions will consume large amounts of browse. These are general statements; and it must be remembered that just because a particular grazing animal consumes and prefers a particular plant in one plant community does not necessarily mean that it will react in a similar manner in another plant community of differing botanical composition. Nevertheless, valuable information can be found regarding the potential of selective grazing for brush suppression in research describing seasonal food habits and dietary preferences of grazing animals.
Juniper and goats: a case study
For many years, Texas’ ranchers have used goats to aid in their efforts to manage brush. Since the 1920’s the Texas A&M University Research Station, located between Sonora and Rocksprings, has been conducting research on the effectiveness of using goats to manipulate vegetation. Results of these studies have revealed that goats spread their grazing pressure more evenly over all kinds of vegetation than do cattle or sheep, which results in light use of grasses (Taylor 1992). This is beneficial for grass vigor and seedling establishment and hence improves the diets of other ruminants. Long-term studies of goat browsing shows dramatic differences between the Research Station grazing treatments, especially with regards to juniper density (Smeins 1990). These studies have shown that goating can significantly reduce juniper seedling recruitment, density and growth rate.
Goats have also compared favorably with all other domestic ruminants in economic production on the Texas A&M Research Station. In a mixed-vegetation complex, their inclusion in the animal mixture has increased efficiency of plant use and helped suppress the growth of brush and extend the life of traditional brush management technologies. Properly managed, the goat is an excellent tool for vegetation manipulation.
The following discussion will address the unique characteristics of goats, the effects of juniper on goats, the role that goats perform in the management of juniper, and present some new ideas on how to improve the goat’s ability to utilize juniper and hence, its efficiency as a biological control agent for juniper.
Goats are ruminants. Goats are ruminants (animals with four-chambered gastric region and dependent upon a mixture of bacteria within their rumens to digest forage) but they’re not necessarily the same as other large foraging animals such as cattle, sheep or white-tailed deer. Different species of ruminants exhibit unique grazing and foraging behaviors that often result in large differences in diet composition and nutritional value (Huston 1978). Hofmann (1989), in a review based on detailed comparative morphological studies of all portions of the digestive system of 65 ruminant species from four continents, classified ruminants into a system of three overlapping morphophysiological feeding types: concentrate selectors, grass and roughage eaters, and intermediate feeders. Under his classification scheme goats would be grouped as intermediate (opportunistic) feeders while cattle would be classified as grass and roughage eaters and white-tailed deer as concentrate selectors.
Effect of juniper on goats. Junipers contain monoterpenoid oils which are volatile. These oils are composed of terpene compounds which are five-carbon rings with alcohol, ketone, and hydrogen side groups (Fig. 2). The kind of side group makes a big difference in the properties of each oil. Terpenoids are chemicals that are produced by the plant and that have no known metabolic use in the plant (not used for growth), other than defense against herbivores that may eat the plant’s leaves, and as an attractant to specific insect pollinators. The volatile oils in juniper give juniper wood many properties that make it a desirable industrial material. Juniper “cedar” posts are used for fencing because the oils make the wood more resistant to insects, bacteria, and fungi. The oils, because of their volatility, give cedar its strong and lasting characteristic smell. Each plant species that contains volatile oils, has a distinct “fingerprint” of oils. This “fingerprint” or oil composition is commonly used to identify different species of plants. For example, blueberry juniper and redberry juniper each has its own individual monoterpene pattern (Fig. 3).
The terpenoids in juniper affect it’s taste and a number of the animal’s metabolic processes. Taste is the most important sense used by domestic livestock in diet selection. When determining to eat a plant, the animal smells the plant first for recognition, then takes a bite of it. The experience of tasting juniper, or anything else, is actually a complex electrochemical interaction that is enacted instantly. Taste buds are chemical receptors which send electrical signals to the brain regarding the chemistry of the food tasted. Individual chemicals in juniper can serve as electrochemical triggers to these taste buds. Two chemicals of similar but different structure could cause the animal to experience different tastes. The emetic system is the part of the brain which, when signaled, will evoke an experience of nausea in the animal. It is possible that this is the area that is signaled by the volatile oils, leading the animals to feel ill after eating a certain amount of juniper. Which oils are present and in what proportion probably influence how much juniper is consumed by livestock. An example is that blueberry juniper is eaten more readily by goats than redberry juniper, probably because of the characteristic profiles of volatile oils (Riddle et al. 1996, Straka 1993).
Terpenes from both blueberry and redberry juniper are bacteriocidic (destroys bacteria) (Brattsten 1979). As such, they serve as chemical defense mechanisms to discourage destructive herbivory and encourage survival of the plant. Since goats are ruminants, the terpenes in juniper could potentially kill some of the ruminal bacteria. Possibly, this could reduce digestive efficiency and cause digestive upset if enough juniper were consumed.
In reality, juniper in livestock diets may not cause high levels of bacterial death. Generally juniper is consumed in relatively small quantities; and when it is consumed, the total terpenoid concentration is diluted during repeated chewing and rumination.
Once in the gastrointestinal tract, terpenes are likely absorbed to a small degree through the rumen wall and to a larger degree from the small intestine. Terpenes then enter the blood supply and are transported to the liver where they are detoxified by multifunctional oxidase enzyme systems (MFO’s) (Brattsten 1979). MFO’s act somewhat like antibodies, where their actual synthesis and production is in direct response to contact with the toxin itself. This process acts as a positive feedback system where initial contact with terpenes stimulates, or increases the production of more enzymes capable of handling more terpenes. MFO’s add molecular groups that break structural bonds to turn terpenes into more polar, water-soluble compounds. This process allows the terpenes to be harmlessly excreted by the animal through its urine.
Optimizing juniper intake. Since we know that juniper intake is limited by the presence of terpenoids, we can overcome this limitation through two different management schemes. We can manage juniper to reduce terpenoid concentration in the foliage and/or we can manage goats to increase their tolerance of the terpenoids. In order to decrease production of terpenoids in the trees we need to understand why juniper produces this secondary chemical. Terpenoid production is influenced by the species of juniper, individual tree and site (the kind of soil the tree grows on).
Considering site first, we know that in a nutrient-limited environment, its not advantageous for juniper to lose nitrogen and carbon that is tied up in its foliage (i.e., plants growing in nitrogen deficient soils appear to produce increased quantities of terpenes; Bryant et al. 1983, Mihaliak and Lincoln 1985). Physiologically the tree protects its resources with these secondary chemicals that keep the foliage from being browsed off.
A promising area of research is measuring monoterpenoid changes in relation to young vs old plants, or to look at the effect of aging on monoterpenoid production and the resulting effects on palatability. Research at the A&M Research Station at Sonora has revealed that monoterpenoid composition for young juniper growth is lower than for old juniper growth and that goats prefer juniper seedlings and regrowth over mature growth. Goats will regularly return to utilize the same juniper trees, harvesting the young regrowth. When the tips are browsed off, regrowth sprouts from lateral buds. This pattern is seen on a larger scale when redberry regrowth sprouts up from a top-killed plant. When these observations were tested in a lab, we found that physiological age of the leaf material influences the amount and kind of monoterpenoids. Young seedlings and sprouts were much lower in monoterpenoids and more palatable than older plants (Fig. 4).
There appears to be a threshold after which leaf material becomes significantly less palatable as the juniper foliage ages and monoterpenoid composition increases. This has important management implications. If juniper can be maintained below this threshold with control methods such as fire, consumption by goats can be increased. Combinations of mechanical treatments, fire, and goating are also beneficial in that they keep juniper canopies within reach of goats and help maintain a higher goat:juniper ratio which is critical for effective goating, since goats can’t consume large volumes of juniper.
Our second approach to juniper management is to increase the tolerance of goats to the monoterpenoids. Monoterpenes are thought to deter goat browsing of juniper plants by being toxic or by reducing nutrient assimilation, or by influencing forage selection at subtoxic levels by imposing high detoxification costs post absorption (increases nutrient demand of animals in order to neutralize terpenoids) ( Freeland and Janzen 1974, Guglielmo et al. 1996). Because of this additional demand for nutrients, adequate nutrition is important to meet the demands of detoxification.
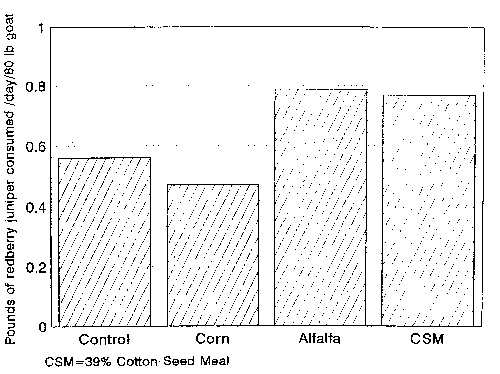
A protein rather than energy supplement appears to be more beneficial to goats consuming juniper. Feeding goats cotton seed meal and alfalfa as a supplement increased redberry juniper intake 40% compared to goats fed a corn supplement and 30% greater for goats in the control treatment (no supplement) in feeding trials on the Sonora Research Station (Fig. 5).
Even though juniper intake can be significantly increased by feeding a protein supplement, individual goat consumption is still relatively low (.8 lbs/hd/day maximum intake for an 80 lb goat). For example, if an average 3-foot-high juniper tree has 10 pounds of consumable foliage and an 80 pound goat consumes .8 lbs/hd/day then it would take over 12 days for all of the foliage to be consumed. Multiply that by 200 to 300 trees per acre and it would take a number of goats to have a major impact on the juniper. Obviously, early treatment of juniper is critical for effective biological control.
Previous research at the Sonora Research Station has shown that Spanish goats have the potential to consume more juniper than Angora goats (Riddle et al. 1996). Further work in this area has determined that goats crossed with the Ibex breed (wild goats) consume more juniper than Spanish goats. We believe this idea of selecting goats for increased juniper consumption has merit. It’s a new approach to livestock management (i.e., manipulate diet selection through selective breeding). This would create a more “ecologically-friendly” animal and allow land managers to increase the harvest efficiency of noxious plants that cause deterioration of the range resource.
Conclusions
Our approach to increasing the efficiency of goating for juniper management involves decreasing terpenoid concentration in the plant and increasing tolerance of these terpenoids in the animals. Listed below are suggestions that may help you to achieve more effective “goating” for juniper management:
1) We recommend frequent browsing with goats to take advantage of the window of palatability that seedlings and regrowth experience before they cross over the threshold and become less palatable.
2) Don’t over stock your range: Year-round goating does not mean year-round overgoating. Use of desirable browse plants should be monitored.
3) Increase grazing pressure (concentration of goats) on target pastures in the winter. Hit the juniper hard when goats will most likely consume it and harm to other plant species can be minimized (i.e., when the warm-season grasses are dormant). Also, initiate a close monitoring program for early detection of juniper seed germination and seedling emergence. Concentrate goats on the young seedlings to attack the juniper when its in its most vulnerable life stage and lowest in terpenoids.
4) Provide a high-quality protein supplement. Adequate nutrition is important to meet the demands of detoxification.
5) Goating alone will generally not solve a juniper problem. A sustainable juniper management system must be developed for each situation. This may include a combination of treatments (i.e., mechanical, fire, chemical and goating). Goating should be viewed as one component of the overall juniper management system. Goating is a unique management tool in that it can directly generate income in the short term to help pay for the other methods and to extend the effective treatment life of the more expensive, conventional control methods.
6) Finally, goating will be most effective where juniper density and biomass are very low because of the limited intake of juniper leaves.

Figure 1. Process by which post-ingestive feedback alters plant palatability and diet selection (modified from Launchbaugh et al. 1997).

Figure 3. Mean concentrations (ug/ml) of terpenes extracted from samples of blueberry and redberry juniper.
Table 1. Management skill required, ease of implementation, comparative costs, and precision of various brush management methods1.
| Brush Control Method | Management Skill | Ease of Implementation | Comparitive Costs | Precision of Method |
| Mechanical | 1 | 1 | 4 | 1 |
| Chemical | 2 | 3 | 3 | 2 |
| Fire | 4 | 4 | 2 | 4 |
| Biological | 3 | 2 | 1 | 3 |
1Values in each column range from 1 to 4. The larger the value, the greater the skill required; difficulty in implementation increases; costs increase; and, precision of method decreases, respectively. In general, these are estimated for the Edwards Plateau region of Texas.
Literature Cited
Archer, S., C.J. Scifres and C.R. Bassham. 1988. Autogenic succession in a subtropical savanna: conversion of grassland to thorn woodland. Ecol. Monogr. 58pp.
Bernays, E.A. and J.C. Lee. 1988. Food aversion learning in the polyphagous grasshopper Schistocerca americana. Pysiol. Entomol. 13:131- 137.
Brattsten, L.B. 1979. Biochemical defense mechanisms in herbivores against plant allelochemicals. In: Herbivores, Their interaction with secondary plant metabolites (G.A. Rosenthal, D.H. Janzen, eds.), pp. 199-270. Academic Press 1979.
Bryant, J.P., F.S. Chapin and D.R. Klein. 1983. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 20:357-368.
Cheek, P.R. and L.R. Shull. 1985. Natural Toxicants in Feeds and Poisonous Plants. AVI Publishing Company, INC. WestPort, Connecticut. 492 pp.
Freeland, W.J., and D.H. Janze. 1974. Strategies in herbivory by mammals: the role of plant secondary compounds. American Naturalist 108:269-289.
Fuhlendorf, S., and F.E. Smeins. 1997. Long-term importance of grazing, fire, and weather patterns on Edwards Plateau vegetation change. In: C.A. Taylor, Jr. (ed) 1997 Juniper Symposium. Tex. Agr. Exp. Stn. Tech. Rpt. 97-1. San Angelo.
Garcia, J. 1989. Food for Tolman: Cognition and cathexis in concert. pp 45-85. In: T. Archer and L. Nilsson. (eds) Aversion, avoidance and anxiety. Lawrence Erlbaum and Associates, Hillsdale, N.J.
Guglielmo, C.G., W.H. Karasov, and W.J. Jakubas. 1996. Nutritional costs of a plant secondary metabolite explain selective foraging by ruffed grouse. Ecology 77:1103-1115.
Hofmann, R.R. 1989. Evolutionary steps of ecophysiological adaptation and diversification of ruminants: a comparative view of their digestive system. Oecologica 78:443-457.
Huston, J.E. 1978. Forage utilization and nutrient requirements of the goat. J. Dairy Sci. 61:988-993.
Johnsen, T.N. 1962. One-seed juniper invasion of northern Arizona grassland. Ecol. 32:187-207.
Launchbaugh, K., C.A. Taylor, Jr., E. Straka, and R. Pritz. 1977. Juniper as forage: an unlikely candidate? In: C.A. Taylor, Jr. (ed) 1997 Juniper Symposium. Tex. Agr. Exp. Sta. Rpt. 97-1. San Angelo.
McPherson, G. R. and H.A. Wright. 1990. Establishment of Juniperus pinchotii in western Texas: environmental effects. J. Arid Environ. 19:283-287.
Mihaliak, C.A. and D. E. Lincoln. 1985. Growth pattern and carbon allocation to volatile leaf terpenes under nitrogen-limiting conditions in Heterotheca subaxillaris (Asteraceae). Oecologia 66:423-426.
Provenza, F.D. 1995. Postingestive feedback as an elementary determinant of food preference and intake in ruminants. J. Range Manage. 48:2-17.
Riddle, R.R., C.A. Taylor, Jr., M.M. Kothmann, and J.E. Huston. 1996. Volatile oil contents of ashe and redberry juniper and its relationship to preference by Angora and Spanish goats. J. Range Manage. 49:35-41.
Scifres, C.J. 1980. Brush Management: Principles and Practices for Texas and the Southwest. Texas A&M Univ. Press, College Station, TX. 360 pp.
Smeins, F.E. 1990. Ashe juniper: Consumer of Edwards Plateau rangeland. Texas Agr. Exp. Sta. Tech. Rep. No. 90-1. Sonora Res. Sta., Sonora, Tex.
Straka, E.J. 1993. Preferences for redberry and blueberry juniper exhibited by cattle, sheep and goats. M.S. Thesis. Texas A&M Univ., College Station, Tex.
Taylor, C.A., Jr. 1992. Brush management considerations with goats. In: J.C. Paschal and C.W. Hanselka (Ed.) Proceedings of the International Conference of meat goat production, management and marketing. pp 144-155.
Taylor, C.A. Jr., and M.H. Ralphs. 1992. Reducing livestock losses from poisonous plants through grazing management. J. Range Manage. 45:9-12.
Thurow, T.L. and C.A.Taylor, Jr. 1995. Juniper effects on the water yield of central Texas rangeland. In: R. Jensen (ed). Proceedings of the 24th water for Texas Conference. Austin. pp 657-665.
Vallentine, J.F. 1989. Range Development and Improvements. 3rd edition. Academic Press, Inc. 524 p.
Van Soest, P.J. 1965. Symposium of factors influencing the voluntary intake of herbage by ruminants: voluntary intake in relation to chemical composition and digestibility. J. Anim. Sci. 24:834-843.
Wright, H.A. 1972. Shrub response to fire. In: Wildland Shrubs: Their Biology and Utilization. U.S. Dep. Agr. Serv. Agr. Gen. Tech. Rep. INT-1.
Comments: Dale Rollins, Professor and Extension Wildlife SpecialistUpdated: Mar. 18, 1997