DAVID M. ENGLE, Division of Agricultural Sciences and Natural Resources, Oklahoma State University, Stillwater, OK 74078
Abstract:The oak cover type in the Southern Great Plains includes one shrubland (sand shinnery oak) and four woodlands (cross timbers-Oklahoma, cross timbers-Texas, juniper-oak, and mesquite-oak). In this paper, I focus on the ecological characteristics of the cross timbers, which is an upland forest dominated by post oak and blackjack oak. Oaks are fire resistant, but disturbance by recurrent fire is believed to have historically prevented development of the closed-canopy forest today. Oak kill by herbicides can increase forage production, but resprouting and resistant species make herbicide treatment short-lived unless follow-up treatments are employed. Wildlife abundance and diversity generally increase with the use of herbicides and fire, but some species, especially interior forest birds, respond negatively to habitat fragmentation.
The oak cover type in the Southern Great Plains includes one shrubland (sand shinnery oak) and four woodlands (cross timbers-Oklahoma, cross timbers-Texas, juniper-oak, and mesquite-oak) (Shiflet 1994). Although they differ considerably, the oak cover types share some key ecological and management characteristics. In this paper, I highlight some of the common ecological characteristics of the oak cover types and how these influence management and values. My presentation will draw on my experience in the cross timbers of Oklahoma and research from the eastern deciduous forest, but the principles can be applied to other oak cover types.
The cross timbers occupies nearly 12 million acres of Oklahoma, Texas, and Kansas (SCS 1981), and is the western extension of the Ozark Plateaus, a portion of the oak-hickory ecosystem that lies west of the Mississippi River and south of the Missouri River (Garrison et al. 1977, Barnes 1991) (Fig. 1). As a vegetation type, the cross timbers includes most of the post oak-blackjack oak forest of Oklahoma (Duck and Fletcher 1943) and the western and eastern cross timbers of Texas (Gould 1975, Smeins 1994). The vegetation is naturally dominated by hardwood trees, but timber and wood products are not economically viable enterprises in the region (Byrd et al. 1984). With the exceptions of subdivisions and ranchettes, livestock grazing and recreational leasing are the primary sources of income to landowners.
Ecological characteristics of oaks
Reproduction in established stands is vegetative, so individuals are commonly aggregated into clones, but recruitment occurs in open grassland or canopy gaps via acorn dispersal by animals (Sherwood and Risser 1972, Collins and Klahr 1991). Oak stands are commonly even-aged because of the absence of disturbance (i.e., old growth stands), even-aged seedling recruitment, or root sprouting after trees have been top-killed (Powell and Lowry 1980, Johnson 1993). Of the two dominant oaks, post oak (Quercus stellata Wangenh.) is apparently the more vigorous resprouter after top removal, with the ability to grow 6 feet in only 4 years (Powell and Lowry 1980, Ewing 1983). Post oak is more sensitive to foliage herbicides, with blackjack oak (Q. marilandica Muenchh.) often dominating herbicide-treated stands (Elwell et al. 1974). Blackjack oak is the more aggressive invader of prairies and abandoned cropland, but invasion by both species is aided by previous invasion of shrubs, especially smooth sumac (Rhus copallina L.) (Petranka and McPherson 1979, Ewing 1983). Post oak is usually the more abundant of the two species, but blackjack oak dominates on sandier soils (Johnson and Risser 1972, Hall and McPherson 1980). Rice and Penfound (1959) observed greater mortality of blackjack oak following a severe drought. Perhaps because it is shorter in stature, blackjack oak will sprout more abundantly following fire (Penfound 1968), but blackjack oak is more likely than post oak to decline with recurrent fire (Huddle and Pallardy 1996).
Upland oaks are known for physiological adaptations that promote tolerance of drought and nutrient-poor soils (Abrams 1992). Physiological adaptations that facilitate survival of drought include deep roots, xeromorphic leaves, low water-potential threshold for stomatal closure, and the ability for osmotic adjustment (Abrams 1990). The ability to maintain high photosynthetic rates during drought and to persist on nutrient-poor soils may provide the oaks a competitive advantage over other trees in the cross timbers (Reich and Hinckley 1980). Because oaks are shade intolerant, they will not survive in a closed overstory (Burns and Honkala 1990).
Several characteristics of oaks enhance their survival of frequent fire. The thickness and low specific gravity of the bark of oaks effectively insulate the cambium from heat damage during fire (Spalt and Reifsnyder 1962, Hengst and Dawson 1994). Bark thickness is dependent on tree size and age, so large, mature trees are seldom top-killed by fire (Hengst and Dawson 1994). An immense underground reservoir of stored food and dormant buds concentrated near the root collar allow oaks to resprout from even the most intense surface fire (Johnson 1993, Bond and van Wilgen 1996). A rapid growth rate and elevated growing points in the canopy are additional adaptations that add to the fire resistance of oaks. Conversely, physiological aging reduces sprouting capacity, so old trees may not resprout after top kill (Kozlowski et al. 1991). Recurrent fire actually promotes oak reproduction by seed by killing stems of oak seedlings, which increases the root:shoot ratio of those seedlings that survive by resprouting (Johnson 1993). Understory oaks with higher root:shoot ratios are more competitive when the overstory is removed.
Acorns represent an important food source for a variety of animals including deer, turkeys, squirrels, small mammals, blue jays, and insects. As a result of heavy seed predation, only about one percent of the acorn crop survive to enter the recruitment pool (Sork 1984). Fire, however, enhances recruitment from acorns by removing excess litter, which increases acorn caching by blue jays and squirrels (Darley-Hill and Johnson 1981, Sander et al. 1983, Johnson and Adkisson 1986) and by reducing acorn predation by insects on the forest floor (Van Lear and Watt 1992). Acorn production in oaks, which can exceed 1,000 lb/ac in the southern Appalachians (Van Lear 1991), is episodic, occurring at 2- to 10-year intervals depending on species (Sander et al. 1983). Acorn production increases with bole diameter, so production in the cross timbers can be minimal on poor sites or in stands of young trees (Goodrum et al. 1971).
Primary productivity and nitrogen cycling
Post oak and blackjack oak have relatively slow growth rates compared to other oaks (Burns and Honkala 1990). Average stand growth (16 ft3/ac/yr) in Oklahoma is less than that defined by USDA Forest Service as a commercially sustainable forest (20 ft3/ac/yr) (Rosson 1994). Diameter growth of the bole is correlated with rainfall and previous growth rate (Johnson and Risser 1973). Above-ground primary production in an immature stand in south central Oklahoma was estimated at 8,300 lb/ac/yr, with >99% of this contributed by post oak and blackjack oak (Johnson and Risser 1974). Leaf dynamics represent an important flow of materials in the ecosystem. Litterfall can exceed 50 lb/ac/day for about a month after the first killing frost (Johnson and Risser 1974). Unlike other oak forests, the litterfall is bimodal, with a second peak in early spring provided by blackjack oak and post oak saplings (Dyksterhuis 1948, Johnson and Risser 1974).
The cross timbers is nitrogen-rich with the potential for redistributing nitrogen to enhance utilization by grazing and browsing animals. Total ecosystem nitrogen in a closed-canopy forest is about 5,000 to 6,000 lb/ac, which can be manipulated with selective herbicides and redistributed to herbaceous plants (Johnson and Risser 1974, Gay et al. 1997) (Table 1). Nitrogen loss via nitrate leaching is possible after tree kill with herbicides because recruitment of herbaceous plants lags peak nitrogen mineralization by 1 to 2 years (Gay et al. 1996). Understory herbage production increases dramatically several years after tree kill. Without overseeding, production will then decline rapidly as a result of nitrogen depletion via nitrate leaching and competition from herbicide-resistant woody plants (Engle et al. 1991, Gay et al. 1996) (Fig. 2).
Soils and vegetation
Some form of moisture compensation is necessary for oaks to compete in the subhumid and semi-arid Great Plains. Trees in the cross timbers occur on sandy soils in a climate that is marginal for tree survival (Fig. 3). Oaks can not survive the droughts common in July and August on the fine-textured soils of adjacent and interspersed grasslands. Thus, within the cross timbers, deep coarse-textured soils support larger trees, shallower coarse-textured soils support smaller trees, and fine-textured, droughty soils support grassland. The heterogeneity of the soils in much of the cross timbers reflect the interbedded sandstones and shales from which the soils are derived. The result is a mosaic of grassland and low-stature upland forest dissected by bottomland forest in alluvial soils along drainages (Ewing et al. 1984).
Relatively low annual rainfall of about 25 to 40 inches, together with sandy, low-fertility soil, accounts for the reduced diversity of trees in the cross timbers compared to elsewhere in the oak-hickory forest (Risser and Rice 1971). The tree component of the cross timbers is dominated by an overstory of post oak and blackjack oak. The understory contains a mixture of other trees, various shrubs, vines, and herbaceous species in varying proportions depending on fire history (Bruner 1931, Penfound 1963, Rice and Penfound 1959, Dwyer and Santelmann 1964, Ewing et al. 1984, Engle et al. 1996a). In virgin grassland interspaces, the herbaceous vegetation resembles mixed prairie or tallgrass prairie, whereas little bluestem (Schizachyrium scoparium [Michx.] Nash) usually dominates the sparse herbaceous understory of the closed-canopied areas.
Vegetation dynamics
Fire. The original character of the cross timbers was likely a mosaic of grassland, savanna-like grassland and oak mottes, oak thickets, and dense woodlands (Rice and Penfound 1959, Penfound 1962, Johnson and Risser 1975, Smeins 1994). Grimm (1984) called this a “fire probability pattern” that resulted from frequent fires superimposed on landscape features that included fire-prone topographic positions as well as natural fire barriers. Periodic intense fire increased the density of oak stems on upland sites (Dyksterhuis 1948, Harlan 1958), but also promoted understory vegetation similar to prairie (Axelrod 1985, Abrams 1992). Early travelers through the region cursed the burned-through vegetation as a formidable obstacle to travel (Irving 1835). In his classic treatment of the cross timbers, E.J. Dyksterhuis (1948) concluded that fires were common before European settlement and that the cross timbers should be regarded as savanna.
Fuel accumulation on the more mesic sites may have produced fires of sufficient intensity to maintain the savanna or cause high tree mortality in closed-canopy stands (Rice and Penfound 1959, Dooley and Collins 1984, Anderson and Brown 1986). Larger trees in savannas appear to be quite stable under frequent fire. Anderson and Brown (1983) demonstrated that isolated savanna trees are protected from fire because leaf litter is blown away from under the trees and other fine fuels do not accumulate because of competition.
The dense stands of oaks and the meager understory forage supply associated with the cross timbers today are unlike the vegetation before settlement by Europeans. Fire exclusion, coupled with livestock grazing, is the primary factor cited in conversion of presettlement oak savannas into closed canopy forest (Rice and Penfound 1959, Johnson and Risser 1975, Abrams 1992). The development of a closed canopy of trees further reduced the likelihood of fuel accumulations to support intense fire (Box 1967, Ehrenreich and Crosby 1960, Anderson and Brown 1986). Johnson and Risser (1975) concluded that the current vegetation is, at least in part, a result of a sequence of events that is difficult to reverse. With their conclusion as a hypothesis, Engle et al. (1996a) investigated the effects of disturbance history on fire behavior and fire effects in three stands in the cross timbers. Fire behavior differed considerably among stands (Fig. 4), but the woody plant community was related more to stand structure and fire frequency than to fire season or fire behavior (Fig. 5). Clearly, as deciduous tree cover increases, the structure and moisture content of fine fuels change, which can reduce the frequency and intensity of fire and the influence of fire on overstory vegetation (Streng and Harcombe 1982, Anderson and Brown 1986). Johnson and Risser (1975) reported a late winter fire in the cross timbers of southern Oklahoma killed trees up to 3.5 inches dbh in an open stand of trees, but damaged very few stems over 1 inch dbh in a closed stand.
That fire can maintain an open stand of hardwoods (Johnson 1993) does not necessarily mean that fire can create an open stand. Following thinning of hardwoods in southeastern Oklahoma, fire was an effective tool for further reducing the oak overstory (Masters 1991). After about a decade of annual burning after leaf drop in the fall or winter, canopy gaps have formed in a previously closed forest in south central Oklahoma, and production in the herbaceous layer is increasing (Russell Stevens, pers. comm.). Similar effects have been observed in the northeastern cross timbers as well (T.G. Bidwell, pers. comm.). Savanna (oak woodland) restoration is an important ecosystem management concern in the midwestern states because of the decline of biodiversity and loss of critical habitat that accompanies closing of the forest canopy (Johnson 1993). Because fire after thinning selectively top-kills small-diameter hardwoods, the thinned stand is maintained. Again, pretreatment community structure and composition influence the effects of burning (White 1986).
Numerous studies in the southeastern pine forests indicate that summer fire more effectively reduces hardwoods than winter fire (e.g., Grano 1970, Grelen 1975, Waldrop et al. 1987, Boyer 1990, Glitzenstein et al. 1995). In the Ouachita Highlands of western Arkansas, fire intensity was more important than season of burning in reducing hardwoods (Sparks et al. 1996). Regardless, it is clear that a single prescribed fire (i.e., not a wildfire in extreme conditions) will not restore an open stand and that frequent fires are necessary to open the overstory canopy (White 1986, Tester 1989, Olson and Platt 1995, Abrahamson and Abrahamson 1996). Even after years of burning, the stands may differ considerably from presettlement stands (White 1986).
Although oaks in the cross timbers appear to be a stable late-successional type (Dooley and Collins 1984), replacement by shade-tolerant, fire-intolerant trees is possible. The increase in overstory juniper, especially eastern redcedar (Juniperus virginiana L.), in the absence of fire poses an increasing threat to the integrity of the cross timbers. Oaks are colonizers, and unless disturbed, the same oak species does not generally succeed itself (Kessler 1992). Eastern redcedar is a shade-tolerant species and can persist for many years as an understory species. Eastern redcedar is a nonsprouting woody plant that is especially susceptible to fire in the seedling and sapling stage, but becomes resistant with increasing size. Winter fires in dry leaf litter of closed forests are of sufficient intensity to kill >80% of eastern redcedar <5 ft in height, but few if any trees taller than 8 ft (Engle and Stritzke 1995). Unfortunately, most of the cross timbers is not burned (Engle et al. 1996b).
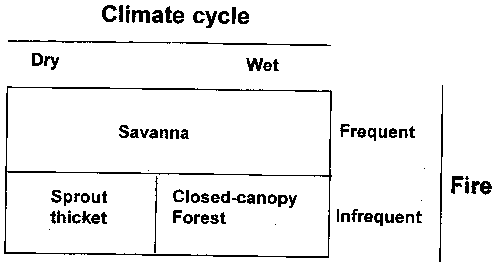
Climate variation significantly influences the structure and extent of woody cover in the cross timbers and may interact with the influence of fire (Dyksterhuis 1948, Rice and Penfound 1959, Johnson and Risser 1975, Abrams 1992). Drought-induced canopy gaps occur periodically throughout the eastern deciduous forest and have an influence on forest structure for decades (Clinton et al. 1993). Furthermore, tree mortality in drought is greater for older trees. Conversely, canopy cover increases in periods of years with above-normal precipitation. Because of favorable precipitation, canopy cover increased from about 140% to almost 200% in just 7 years in north central Oklahoma (J.F. Stritzke, unpublished data). In the absence of other anthropogenic disturbance, savanna is probably stable in either wet or dry cycles under frequent fire regimes, but sprout thickets or closed-canopy forest develop under infrequent fire depending on climate cycle (Fig. 6). Herbicides.Herbicides have been used on closed-canopy stands in the cross timbers to release understory forages from competition with the overstory oaks. Vegetation management in the cross timbers in this context can be looked at as inducing regression with the objective of deriving a grassland on a site with a natural potential to produce trees (Fig. 7). Follow-up treatments are required to maintain the derived grassland after the herbicide treatment of the overstory oaks. With no follow-up brush control treatments, most sites in the cross timbers will eventually become dominated by resprouts and herbicide-resistant woody plants. It is important to recognize that oak resprouts are more difficult to kill with herbicides than older trees.
Significant increases in production of herbaceous plants follow reductions of the overstory hardwoods in the cross timbers and related oak-hickory forests. One acre of land cleared of trees in Missouri produced as much herbaceous forage as 40 to 60 acres of hardwood forest (Ehrenreich and Buttery 1960). In the Ouachita Highlands of eastern Oklahoma, forage production increased from less than 100 lb/ac to more than 1,500 lb/ac in three years with a single application of 2,4,5-T (Stritzke et al. 1975). Similar increases in production have been reported in Texas and Oklahoma with tebuthiuron, a soil-applied herbicide used for brush control (Scifres et al. 1981, Engle et al. 1991).
A major concern of herbicide-based vegetation management strategies in the cross timbers is the secondary brush associated with sprout thickets. Secondary brush is the result of oak resprouting, release of herbicide-resistant trees, and invasion of other trees. Secondary brush is usually a more immediate problem with foliar applied herbicides than soil applied herbicides because herbicide deposition decreases significantly below the overstory trees with foliar applied herbicides. The soil-applied herbicide tebuthiuron has fewer immediate secondary brush problems, but when juniper is present in the understory, a juniper forest may eventually dominate. Follow-up treatment is necessary because secondary brush will eventually cause forage production to decline to pretreatment levels or below. Follow-up herbicide treatments are expensive and often less effective than the initial herbicide treatment. Integrating prescribed fire into vegetation management may offer a low-cost alternative for deriving grasslands and for extending the benefits of the initial herbicide treatment. Burning can suppress oak resprouts (Stritzke et al. 1975, Stritzke et al. 1991) and nearly eliminate non-sprouting juniper (Engle and Stritzke 1995).
Influence of vegetation management on wildlife
Vegetation in the cross timbers can be managed through fire and herbicides to structure more suitable habitats for certain species of wildlife and to improve nutritional quality and production of herbaceous and woody forages. Many nongame birds are habitat specialists, so although herbicides create more diverse habitats that support greater bird diversity and abundance, some interior woodland species are found only on untreated stands (Schulz et al. 1992a, Schulz et al. 1992b). Fire wounds initiate heart rot that creates cavities in oaks that benefits a variety of wildlife species including cavity-nesting birds (Brawn et al. 1982). Both density and body condition of cottontail rabbits increases in response to higher quality and quantity of forages when the oak overstory is removed and a more open habitat is created (Lochmiller et al. 1991, Lochmiller et al. 1995). Habitat quality for white-tailed deer improves with removal of oak overstory, and is optimized with herbicides (e.g., triclopyr) that leave understory woody species important for nutritious browse and horizontal cover (Soper et al. 1993, Leslie et al. 1996). The influence of herbicides and fire on wildlife habitat in the cross timbers is transitory, so maintaining a particular habitat structure and composition requires maintenance treatments.
The more profound impact on wildlife communities in the cross timbers is through permanent cover type conversions. Many conversions are the result of changes in land use in the wildland-urban intermix. Landscapes in northeastern Oklahoma declined in quality with the replacement of native vegetation by cropland and introduced pasture when large ranches were divided into smaller ranchettes (Boren et al. 1997). The result was an increase in habitat generalists and loss of neotropical migrant birds (Boren 1996).
Table 1. Total nitrogen and biomass in herbaceous and understory woody plants in the cross timbers of north central Oklahoma 1 1/2 years following herbicide application (from Gay et al. 1997).
| Treatment1 | Total N | Biomass |
| ———————– | (lb/ac)——————- | |
| Control | 2 | 290 |
| No seed | 24 | 1,650 |
| Tall fescue | 60 | 3,140 |
| Old World bluestem | 31 | 2,760 |
1Treatments are: control = no herbicide and no overseeding; no seed = herbicide with no overseeding; fescue = herbicide with tall fescue overseeding; Old World bluestem = herbicide with Old World bluestem overseeding.

Figure 1. The cross timbers (solid) is the western extension of the Ozark Plateaus (cross-hatched), a portion of the oak-hickory ecosystem (after Garrison et al. 1987, SCS 1981).

Figure 2. Production of understory grasses and forbs after tree kill with herbicide in the cross timbers (Engle and Stritzke 1991, D.M. Engle unpublished data).

Figure 3. Potential vegetation as a function of soil texture and climate (adapted from Harlan 1958).
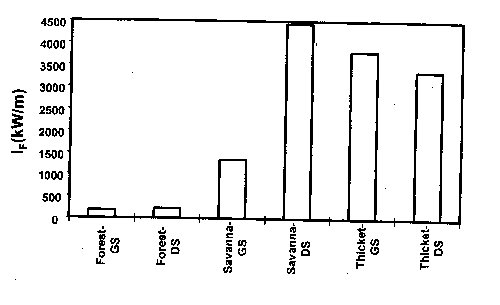
Figure 4. Fireline intensity of fires in three stands of cross timbers and in two seasons (GS=growing season, DS=dormant season).
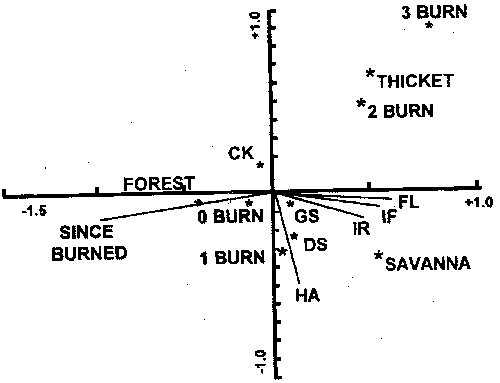
Figure 5. Uniplot of centroids of envionmental variables resulting from canonical correspondence analysis of woody plant cover and fire environment (after Engle et al. 1996a). Fire behavior variables: FL=flamelength, IF=fireline intensity, HA=heat per unit area, IR=reaction intensity; fire frequence variables: 0BURN=no burns since 1993; 1BURN=one burn since 1993, 2BURN=two burns since 1993, 3BURN=three burns since 1993, SINCE=number of years in 1993 since the previous burn; stand structure: resprout thicket (THICKET), closed forest (FOREST), and open woodland (SAVANNA). Environmental variables more distant from the origin of the axes explain more of the variation in woody plant cover.

Figure 7. Vegetation dynamics in the cross timbers are driven by herbicides and fire. Vegetation management in the cross timbers requires a long-term management strategy aimed at preventing succession to upland hardwood forest. Because grassland is a lower successional stage than forest, periodic treatment is required to maintain the grassland aspect.
Literature Cited
Abrahamson, W.G., and C.R. Abrahamson. 1996.Effects of fire on long-unburned Florida uplands. J. Veg. Sci. 7:565-574.
Abrams, M.D. 1990. Adaptations and responses to drought in Quercus species of North America. Tree Physiol. 7:227-238.
Abrams, M.D. 1992. Fire and the development of oak forests. BioScience 42:346-353.
Anderson, R.C., and L.E. Brown. 1983. Comparative effects of fire on trees in a midwestern savannah and an adjacent forest. Bull. Torrey Bot. Club 110:87-90.
Anderson, R.C., and L.E. Brown. 1986. Stability and instability in plant communities following fire. Amer. J. Bot. 73:364-368.
Axelrod, D.I. 1985. Rise of the grassland biome, central North America. Bot. Rev. 51:163-201.
Barnes, B.V. 1991. Deciduous forests of North America, pp. 218-344. In: E. Rohrig and B. Ulrich. Temperate deciduous forests. Ecosystems of the world 7. Elsevier. New York.
Bond, W.J., and B.W. van Wilgen. 1996. Fire and Plants. Population and community biology series 14. Chapman & Hall. New York. 263 pp.
Boren, J.C. 1995. Effects of agriculture intensification on landscapes and avian community structure. Ph.D. Diss., Oklahoma State Univ. Stillwater.
Boren, J.C., D.M. Engle, M.S. Gregory, R.E. Masters, T.G. Bidwell, and V.A. Mast. 1997. Landscape structure and change in a hardwood forest-tallgrass prairie ecotone. J. Range Manage. 50:244-249.
Box, T.W. 1967. Brush, fire and West Texas rangeland. Tall Timbers Fire Ecol. Conf. Proc. 6:7-19.
Boyer, W.D. 1990. Growing-season burns for control of hardwoods in longleaf pine stands. USDA For. Serv. Res. Pap. SO-256. 7 pp.
Brawn, J.D., W.H. Elder, and K.E. Evans. 1982. Winter foraging by cavity-nesting birds in an oak-hickory forest. Wildl. Soc. Bull. 10:271-275.
Bruner, W.E. 1931. The vegetation of Oklahoma. Ecol. Monogr. 1:99-188.
Burns, R.J., and B.H. Honkala. 1990. Silvics of North America. Vol. 2. Hardwoods. USDA Agr. Handbook 654. Washington, D.C.
Byrd, N.A., C.E. Lewis, and H.A. Pearson. 1984. Management of southern pine forest for cattle production. USDA Forest Serv. Gen. Rep. R8-GR4.
Clinton, B.D., L.R. Boring, and W.T. Swank. 1993. Canopy gap characteristics and drought influences in oak forests of the Coweeta Basin. Ecology 74:1551-1558.
Collins, S.L., and S.C. Klahr. 1991. Tree dispersion in oak-dominated forests along an environmental gradient. Oecologia 86:471-177.
Darley-Hill, S., and W.C. Johnson. 1981. Acorn disposal by the blue jay (Cyanocitta cristata). Oecolgia 50:231-232.
Dooley, K.L., and S.L. Collins. 1984. Ordination and classification of western oak forests in Oklahoma. Amer. J. Bot. 71:1221-1227.
Duck, L.G., and J.B. Fletcher. 1943. A game type map of Oklahoma. Div. Wildlife Restoration, Oklahoma Game and Fish Dept. Oklahoma City. 1 p. Dwyer, D.D. and P.W. Santelmann. 1964. A comparison of post oak-blackjack oak communities on two major soil types in North Central Oklahoma. Oklahoma Agr. Exp. Sta. B-626. 15 pp.
Dyksterhuis, E.J. 1948. The vegetation of the western cross timbers. Ecol. Monogr. 18:327-376.
Ehrenreich, J.H. and R.F. Buttery. 1960. Increasing forage on Ozark wooded range. Central States Forest Exp. Sta. Tech. Rep. 177.
Ehrenreich, J.H. and J.S. Crosby. 1960. Forage production on sprayed and burned areas in the Missouri Ozarks. J. Range Manage. 13:68-70.
Elwell, H.M., P.W. Santelmann, J.F. Stritzke, and H. Greer. 1974. Brush control research in Oklahoma. Okla. Agr. Exp. Sta. B-712. 46 pp.
Engle, D.M., T.G. Bidwell, and R.E. Masters. 1996a. Restoring cross timbers ecosystems with fire. Pages 190-199 in Trans. 61st No. Amer. Wildl. and Natur. Resour. Conf.
Engle, D.M., T.G. Bidwell, and M.E. Moseley. 1996b. Invasion of Olahoma rangelands and forests by eastern redcedar and ashe juniper. Oklahoma Coop. Ext. Serv. Circular E-947. 10 pp.
Engle, D.M., and J.F. Stritzke. 1995. Fire behavior and fire effects on eastern redcedar in hardwood leaf-litter fires. Int. J. Wildland Fire 5:135-141.
Engle, D.M., J.F. Stritzke, and F.T. McCollum. 1991. Vegetation management in the cross timbers: response of understory vegetation to herbicides and burning. Weed Tech. 5:406-410.
Ewing, A.L. 1983. Oak stand age and size structure after fifty years succession in an Oklahoma upland forest. Unpublished manuscript. Dept. Agronomy. Oklahoma State University. Stillwater. 19 pp.
Ewing, A.L., J.F. Stritzke, and J.D. Kulbeth. 1984. Vegetation of the Cross Timbers Experimental Range, Payne County, Oklahoma. Oklahoma Agr. Exp. Sta. Res. Rep. P-856. 40 pp.
Garrison, G.A., A.J. Bjugstad, D.A. Duncan, M.E. Lewis, and D.R. Smith. 1977. Vegetation and environmental features of forest and range ecosystems. Agr. Handb. No. 475, USDA Forest Serv. U.S. Government Printing Office, Washington, D.C.
Gay, D.L., E.R. Allen, D.M. Engle, and J.F. Stritzke. 1996. Nitrate dynamics following brush control in a post oak-blackjack oak forest. Agron. J. 88:536-540.
Gay, D.L., D.M. Engle, E.R. Allen, and J.F. Stritzke. 1997. Nitrogen and biomass dynamics following brush control in the cross timbers. J. Range Manage. 50:55-61.
Glitzenstein, J.S., W.J. Platt, and D.R. Streng. 1995. Effects of fire regime and habitat on tree dynamics in north Florida longleaf pine savannas. Ecol. Monogr. 65:441-476.
Goodrum, P.D., V.H. Reid, and C.E. Boyd. 1971. Acorn yields, characteristics, and management criteria of oaks for wildlife. J. Wildl. Manage. 35:520-532.
Gould, F.W. 1975. Texas plants, a checklist and ecological summary. Third edition. Texas Agr. Exp. Sta. MP-585/revised. Texas A&M Univ. College Station. 121pp.
Grelen, H.E. 1975. Vegetation response to twelve years of seasonal burning on a Louisiana longleaf pine site. USDA For. Serv. Res. Note SO-192. 5 pp.
Grimm, E.C. 1984. Fire and other factors controlling the Big Woods vegetation of Minnesota in the mid-nineteenth century. Ecol. Monogr. 54:291-311.
Hall, S.L., and J.K. McPherson. 1980. Geographic distribution of two species of oaks in Oklahoma in relation to seasonal water potential and transpiration rates. Southwest. Natur. 25:283-295.
Harlan, J.R. 1958. Grasslands of Oklahoma. Oklahoma State Univ. Processed Teaching Manual. Agron. Dept. 160 pp.
Hengst, G.E., and J.O. Dawson. 1994. Bark properties and fire resistance of selected tree species from the central hardwood region of North America. Can. J. For. Res. 24:688-696.
Huddle, J.A., and S.G. Pallardy. 1996. Long-term annual and periodic burning on tree survival and growth in a Missouri Ozark oak-hickory forest. Forest Ecol. and Manage. 82:1-9.
Irving, W. 1835. A Tour of the Prairies. Reprinted 1926. Harlow Publishing. Okla. City. 252 pp.
Johnson, C.W., and C.S. Adkisson. 1986. Airlifting the oaks. Natural History 95:40-47.
Johnson, F.L., and P.G. Risser. 1972. Some vegetation-environment relationships in the upland forests of Oklahoma. J. Ecol. 60:655-663.
Johnson, F.L., and P.G. Risser. 1973. Correlation analysis of rainfall and annual ring index of central Oklahoma blackjack and post oak. Amer. J. Bot. 60:475-478.
Johnson, F.L., and P.G. Risser. 1974. Biomass, annual net primary production, and dynamics of six mineral elements in a post oak-blackjack oak forest. Ecology 55:1246-1258.
Johnson, F.L. and P.G. Risser. 1975. A quantitative comparison between an oak forest and an oak savannah in Central Oklahoma. Southwest. Natur. 20:75-84.
Johnson, P.S. 1993. Perspectives on the ecology and silvaculture of oak-dominated forests in the central and eastern states. USDA Forest Serv. Gen. Tech. Rep. NC-153.
Kozlowski, T.T., P.J. Kramer, and S.G. Pallardy. 1991. The physiological ecology of woody plants. Academic Press. San Diego, Calif. 657 pp.
Leslie, D.M., Jr., R.B. Soper, R.L. Lochmiller, and D.M. Engle. 1996. Habitat use by white-tailed deer on cross timbers rangeland following brush management. J. Range Manage. 49:401-405.
Lochmiller, R.L., J.F. Boggs, S.T. McMurry, D.M. Leslie, Jr., and D.M. Engle. 1991. Respose of cottontail rabbit populations to herbicide and fire applications on cross timbers rangeland. J. Range Manage. 44:150-155.
Lochmiller, R.L., D.G. Pietz, S.T. McMurry, D.M. Leslie, Jr., and D.M. Engle. 1995. Alterations in condition of cottontail rabbits (Sylvilagus floridanus) on rangelands following brush management. J. Range Manage. 48:232-239.
Masters, R.E. 1991. Effects of fire and timber harvest on vegetation and cervid use on oak-pine sites in Oklahoma Ouachita Mountains. Pages 168-176 in S.C. Nodvin and T.A. Waldrop (Eds.) Fire and the environment: Ecological and cultural perspectives. Proc. Inter. Symp. USDA For. Serv. Gen. Tech. Rep. SE-69.
Olson, M.S., and W.J. Platt. 1995. Effects of habitat and growing season fires on resprouting of shrubs in longleaf pine savannas. Vegetatio 119:101-118.
Penfound, W.T. 1962. The savanna concept in Oklahoma. Ecology 43:774-775.
Penfound, W.T. 1963. The composition of a post oak forest in southcentral Oklahoma. Southwest Natur. 8:114-115.
Penfound, W.T. 1968. Influence of a wildfire in the Wichita Mountains Wildlife Refuge, Oklahoma. Ecology 49:1003-1006.
Perino, J.V., and P.G. Risser. 1972. Some aspects of structure and function in Oklahoma old field succession. Bull. Torrey Bot. Club 99:233-239.
Petranka, J.W., and J.K. McPherson. 1979. The role of Rhus copallina in the dynamics of the forest-prairie ecotone in north-central Oklahoma. Ecology 60:956-965
Powell, J., and D.P. Lowry. 1980. Oak (Quercus spp.) sprouts growth rates on a central Oklahoma shall savannah range site. J. Range Manage. 33:312-313.
Reich, P.B., and T.M. Hinckley. 1980. Water relations, soil fertility, and plant nutrient composition of a pygmy oak ecosystem. Ecology 61:400-416.
Rice, E.L., and W.T. Penfound. 1959. The upland forests of Oklahoma. Ecology 40:593-608.
Risser, P.G., and E.L. Rice. 1971. Diversity of tree species in Oklahoma upland forests. Ecology 49:1006-1009.
Rosson, J.F., Jr. 1994. Quercus stellata growth and stand characteristics in the Quercus stellata-Quercus marilandica forest type in the cross timbers region of central Oklahoma. Page 329-333 in J.S. Fraslish, R.C. Anderson, J.E. Ebinger, and R. Szafoni (Eds.). Proc. North Amer. conf. on barrens and savannas. USDA Forest Serv. Southern Forest Exp. Sta. Proc. Rep.
Sander, I.L., C.E. McGee, K.G. Day, and R.E. Willard. 1983. Oak hickory. Pages 116-120 in R.M. Burns (Comp.). Silvacultural systems for the major forest types of the United States. USDA Forest Serv. Agr. Handbook No. 445. Washington, D.C.
Schulz, C.A., D.M. Leslie, Jr., R.L. Lochmiller, and D.M. Engle. 1992a. Autumn and winter bird populations in herbicide-treated cross timbers in Oklahoma. Amer. Midl. Natur. 127:215-223.
Schulz, C.A., D.M. Leslie, Jr., R.L. Lochmiller, and D.M. Engle. 1992b. Herbicide effects on cross timbers breeding birds. J. Range Manage. 45:407-411.
Scifres, C.J., J.W. Stuth, and R.W. Bovey. 1981. Control of oaks (Quercus spp.) and associated woody species on rangeland with tebuthiuron. Weed Sci. 29:270-275.
Sherwood, R.T.B., P.G. Risser. 1979. Dispersion and co-dispersion in Oklahoma upland forests. Bull. Torrey Bot. Club 106:200-204.
Shiflet, T.N. (ed.). 1994. Rangeland cover types of the United States. Society for Range Management. Denver, Colo. 152 pp.
Smeins, F. 1994. Cross timbers–Texas–little bluestem-post oak. SRM 732. Pages 107-108 in T.N. Shiftlet (Ed.). Rangeland Cover Types of the United States. Society for Range Management. Denver.
Soil Conservation Service. 1981. Land resource regions and major land resource areas of the United States. Agr. Handbook No. 296. USDA. U.S. Government Printing Office, Washington, D.C.
Soper, R.B., R.L. Lochmiller, D.M. Leslie, Jr., and D.M. Engle. 1993. Nutritional quality of browse after brush management on cross timbers rangeland. J. Range Manage. 46:399-410.
Sork, V.L. 1984. Examination of seed dispersal and survival in red oak, Quercus rubra (Fagaceae), using metal-tagged acorns. Ecology 65:1020-1022.
Spalt, K.W., and W.E. Reifsnyder. 1962. Bark characteristics and fire resistance: A literature survey. USDA For. Serv. Ocassional Pap. S0-193. 19 pp.
Sparks, J.C., R.E. Masters, and D.M. Engle. 1996. Effects of growing and dormant season prescribed fire on vegetation in red-cockaded woodpecker clusters. Ann. Progress Rep. to USDA For. Serv., Ouachita Nat. Forest. Forestry Dept. Oklahoma State Univ. Stillwater. 67 pp.
Treng, D.R., and P.A. Harcombe. 1982. Why don’t East Texas savannas grow up to forest? Amer. Midl. Natur. 108:278-294.
Stritzke, J.F., W.E. McMurphy, and R.W. Hammond. 1975. Brush control with herbicides: Sarkey’s research and development report. Oklahoma Agr. Exp. Sta. MP-95. 31 pp.
Stritzke, J.F., D.M. Engle, and F.T. McCollum. 1991. Vegetation management in the Cross timbers: Response of woody species to herbicides and burning. Weed Tech. 5:400-405.
Tester, J.R. 1989. Effects of fire frequency on oak savanna in east-central Minnesota. Bull. Torrey Bot. Club 116:134-144.
Van Lear, D.H. 1991. Fire and oak regeneration in the Southern Appalachians. Pages 15-21 in S.C. Nodvid and T.A. Waldrop (Eds.). Fire and the environment: Ecological and cultural perspectives. USDA Forest Serv. Gen. Tech. Rep. SE-69.
Van Lear, D.H., and J.M. Watt. 1992. The role of fire in oak regeneration. Pages 66-78 in D.L. Loftis and C.E. McGee (Eds.). Oak regeneration: Serious problems, practical recommendations. USDA Forest Serv. SE.
Waldrop, T.A., D.H. Van Lear, F.T. Lloyd, and W.R. Harms. 1987. Long term studies of prescribed burning in loblolly pine forests of Southeastern Coastal Plain. USDA For. Serv. Gen. Tech. Rept. SE-45. 23 pp.
White, A.S. 1986. Prescribed burning for oak savanna restoration in central Minnesota. USDA Forest Serv. Res. Pap. NC-266.
Comments: Dale Rollins, Professor and Extension Wildlife SpecialistUpdated: Mar. 18, 1997
Pingback: How to Choose the Right Oak Trees | Garden Style San Antonio
Pingback: Oak Species that are Wilt Resistant | Garden Style San Antonio